«THE EPIGENETIC PROTECTION SHELL OF EUCHROMATIN DNA» by Andreas Johannes Kesel
English | EPUB | 2.2 MB
English | EPUB | 2.2 MB
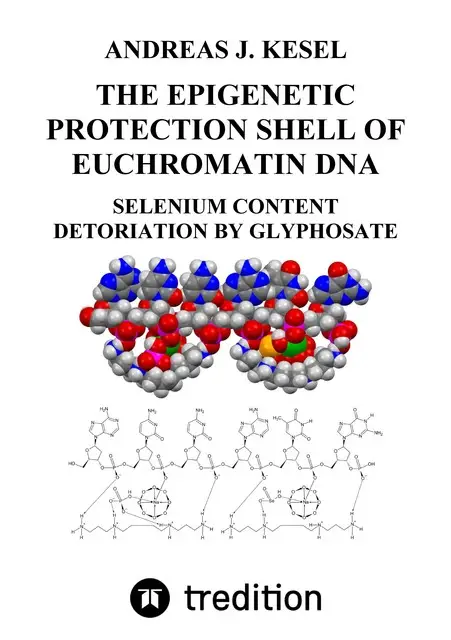
Oxygen exists in two gaseous (dioxygen and ozone) and six solid allotropic modifications. An additional allotropic modification of oxygen, the cyclooctaoxygen, was predicted to exist in 1990. The first synthesis and characterization of cyclooctaoxygen as its sodium crown complex, isolated in the form of three cytosine nucleoside hydrochloride complexes, was reported in 2016. Cyclooctaoxygen sodium was synthesized in vitro from atmospheric oxygen, or catalase effect-generated oxygen, under catalysis of cytosine nucleosides and either ninhydrin or eukaryotic low-molecular weight RNA. Thin-layer chromatographic mobility shift assays were applied on specific nucleic acids and the cyclooctaoxygen sodium complex. The cationic cyclooctaoxygen sodium complex was shown to bind to nucleic acids (RNA and DNA), to associate with single-stranded DNA and spermine phosphate, and to be essentially non-toxic to cultured mammalian cells at 0.1–1.0 mM concentration. It is postulated that cyclooctaoxygen is formed in most eukaryotic cells in vivo from dihydrogen peroxide in a catalase reaction catalysed by cytidine and RNA. A molecular biological model was deduced for a first epigenetic shell of eukaryotic in vivo DNA. This model incorporates an epigenetic explanation for the interactions of the essential micronutrient selenium (as selenite) with eukaryotic in vivo DNA. The sperminium hydrogen phosphate/cyclooctaoxygen sodium complex is calculated to cover the actively transcribed regions (2.6%) of bovine lymphocyte interphase genome. Cyclooctaoxygen seems to be naturally absent in hypoxia-induced highly condensed chromatin, taken as a model for eukaryotic metaphase/anaphase/early telophase mitotic chromatin. Hence, it is proposed that the cyclooctaoxygen sodium-bridged sperminium hydrogen phosphate and selenite coverage serves as an epigenetic shell of actively transcribed gene regions in eukaryotic 'open' euchromatin DNA. It is proposed that the sperminium phosphate/cyclooctaoxygen sodium complex coverage of nucleic acids is essential to eukaryotic gene regulation and promoted proto-eukaryotic evolution. Cyclooctaoxygen sodium-bridged sperminium hydrogen selenite is calculated to serve as a marker shell component at ATG start codons in human euchromatin DNA mRNA genes, both at the translation initiation triplet and at 5′-untranslated region upstream ATGs. The total herbicide glyphosate (ROUNDUP®) and its metabolite (aminomethyl)phosphonic acid (AMPA) are proved to represent 'epigenetic poisons', since they both selectively destroy the cyclooctaoxygen sodium complex. This definition is of reason, since the destruction of cyclooctaoxygen is certainly sufficient to bring the protection shield of human euchromatin into collateral epigenetic collapse. The total herbicide glyphosate and its environmental metabolite (aminomethyl)phosphonic acid (AMPA) can be associated in vitro with catalytic detoriation of eukaryotic euchromatin genetic information. The epigenetic shell of eukaryotic euchromatin is susceptible to decay induced by catalytic epigenetic poisons threatening eukaryotic genomic heritage.