Halo alkanes and Halo arenes
MP4 | Video: h264, 1280x720 | Audio: AAC, 44.1 KHz
Language: English | Size: 1.15 GB | Duration: 2h 13m
MP4 | Video: h264, 1280x720 | Audio: AAC, 44.1 KHz
Language: English | Size: 1.15 GB | Duration: 2h 13m
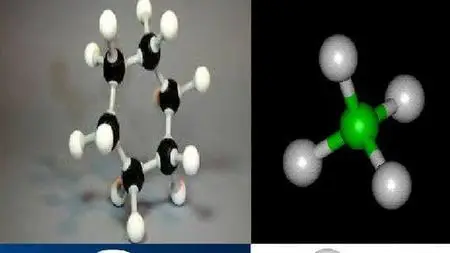
Chloro Methane
What you'll learn
The students will get a wide picture of Organic Compounds.
The students will learn about halo alkanes and halo arenes..
The students will have a deep knowledge about the reaction parameters.
The students will know about the importance of Organic Compounds like chloro methane and chloro benzene.
Requirements
The child should have clear concepts of Organic Compounds and their derivative's.
Description
Alkyl halides or halo alkanes are compounds in which a halogen is bonded to an alkyl group. They have the general formula RX (where R is alkyl group CnH2n+1) X is halogen atom.
Alkyl halides are classified as primary, secondary and tertiary alky halides
depending on whether the halogen atom is attached to a primary, secondary or tertiary carbon atom respectively.
Aromatic halogen compounds or halo arenes are the halogen compounds which contain at least one aromatic ring. Halogen derivatives of aromatic compounds are of two types.
Aryl halides: In this type of compounds, the halogen atom is directly linked to the carbon of benzene nucleus.
Aralkyl halides: In this type of compounds halogen is linked to the carbon atom of the side chain
Boiling Points
The boiling points of haloalkanes are in the order RCl < RBr < RI. It is because with increase in size and mass of halogen atom the magnitude of Vander Waal’s forces of attraction increases. Among isomeric alkyl halides, the boiling point decreases with increase in branching in alkyl group.
Solubility
Haloarenes are insoluble in water, acids or base but are soluble in organic solvents. Haloarenes are insoluble in water because they can not form hydrogen bonds with water molecules.
Density
They are all heavier than water. Their densities follow the order
Iodo > Bromo > Chloro
Who this course is for
For the Beginners and students of twelfth standard.